Background: Despite recent advances, first-line (1L) chemoimmunotherapy (CIT) for patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) is largely unchanged for decades.In the Smart Start trial, we established the feasibility of targeted chemotherapy-free 1L therapy with rituximab (R), lenalidomide (L), and ibrutinib, prior to chemotherapy in patients with newly diagnosed DLBCL with an overall response rate (ORR) and complete response rate (CRR) of 86% and 36%, respectively, prior to chemotherapy, and a 2 year progression free survival rate of 91.3% following 6 cycles of standard CIT (Westin et al, JCO 2023, PMID: 35952327). One patient withdrew from the study after achieving CR and prior to receiving CIT and remains in remission without further therapy at >4 years. BTK inhibitors like acalabrutinib (A) and the immunomodulatory agent L result in synthetic lethality in non-GCB DLBCL models. Both the CD20 antibody R and CD19 antibody tafasitamab (T) demonstrate clinical activity when combined with L in patients with DLBCL. L, T, R, and A are immunomodulatory, driving an anti-tumor immune response. Based upon these data, we are conducting the Smart Stop trial (NCT04978584) to evaluate if the number of CIT cycles can be reduced or omitted after response to targeted therapy, and now report the preplanned interim results from cohort 1.
Methods: Eligibility criteria include patients who are 18y+ with previously untreated DLBCL (initially the Hans algorithm was used to select non-GCB DLBCL, but this criteria was removed this criteria) with adequate organ and bone marrow function. Patients with known CNS involvement of lymphoma, recent thrombosis, or inability to tolerate prophylactic anticoagulation are ineligible.
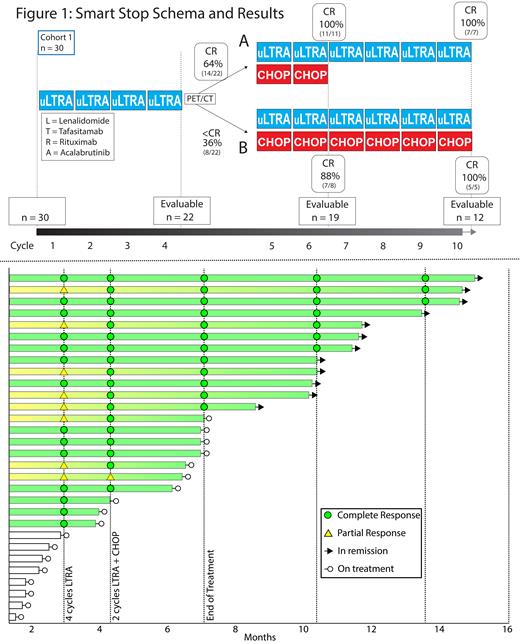
Patients receive lenalidomide 25mg on days (d) 1-10, tafasitamab 12mg/kg IV d1, 8, and 15, rituximab 375mg/m2 IV d1, and acalabrutinib 100mg PO twice/d in a 21 day cycle. All patients receive LTRA for 4 cycles, followed by PET/CT scan (CR defined as a 5PS of 1, 2, or 3). In cohort 1, patients receive an additional 6 cycles of LTRA combined with a response adapted number of cycles of CHOP therapy. After 4 cycles of LTRA, patients with CR receive 2 cycles of CHOP, and all other patients receive 6 cycles of CHOP. In the upcoming cohort 2, patients with CR after 4 cycles of LTRA are planned to receive no cycles of CHOP.
The primary objectives are to determine the 1A: ORR after 4 cycles of uLTRA and 1B: CRR at of LTRA +/-CHOP at the end of therapy.
Results: Cohort 1 enrolled 30 patients from May 2022 - July 2023, with all patients expected to be evaluable for 1A endpoint and 22 patients (73%) evaluable for endpoint 1B by the ASH meeting.
The median age was 61 years (range: 32-85), 30% were >= 70 years, and 50% were female. 67% of patients have poor risk R-IPI, 77% had advanced stage, and 83% had an elevated LDH. 83% had the non-GCB subtype and 17% had the GCB subtype of DLBCL.
After 4 cycles of LTRA, the ORR is 100% and the CRR is 64% (endpoint 1A, n = 14/22 evaluable at abstract submission, figure 1 schema and swimmer plot). After an additional 2 cycles of LTRA-CHOP, the ORR is 100% and the CRR is 95% (n = 18/19 evaluable at abstract submission). At end of all therapy, the CRR is 100% (endpoint 1B, n = 12 evaluable at abstract submission), including 7 patients who received LTRA for 10 cycles and CHOP for 2 cycles (early CR) and 5 patients who received LTRA for 10 cycles and CHOP for 6 cycles. To date, no patient has progressive lymphoma, including the first 6 patients who have 3 and 6 month follow up scans.
47% of patients experienced rash (13% grade 3), and 40% of patients required a dose reduction of lenalidomide.
Conclusions: The Smart Stop trial demonstrates that combination of lenalidomide, tafasitamab, rituximab, and acalabrutinib is highly effective as an initial chemotherapy-free combination in patients with newly diagnosed DLBCL, and may allow for a response adapted reduction in chemotherapy. Response and time to event data will be updated for presentation at the meeting. We will soon open cohort 2 which will enroll an additional 30 patients where those with early CR after 4 cycles of LTRA will receive no cycles of CHOP.
OffLabel Disclosure:
Westin:MonteRosa: Consultancy; Kymera: Research Funding; SeaGen: Consultancy; Abbvie: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; Calithera: Research Funding; Kite/Gilead: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Nurix: Consultancy. Steiner:GSK: Research Funding; Rafael Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding; Seattle Genetics: Research Funding. Jain:AstraZeneca: Consultancy, Honoraria. Iyer:Salarius: Consultancy; Seagen: Consultancy, Research Funding; Yingli: Consultancy, Research Funding; Innate: Research Funding; Merck: Research Funding; CRISPR: Consultancy, Research Funding; CuraBio: Speakers Bureau; Drenbio: Research Funding; American Society of Hematology: Speakers Bureau; American Society of Transplant and Cellular Therapy: Speakers Bureau; Acrotech: Consultancy, Research Funding; Legend: Research Funding; Astra Zeneca: Research Funding; Ono: Research Funding; Pfizer: Research Funding. Nastoupil:AbbVie: Honoraria; ADC Therapeutics: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; Caribou Biosciences: Honoraria, Research Funding; DeNovo: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Regeneron: Honoraria; AstraZeneca: Honoraria; Gilead Sciences/Kite Pharma: Honoraria, Research Funding. Neelapu:Astellas Pharma: Consultancy, Other: Advisory board member; N/A: Patents & Royalties: Related to cell therapy and the safety switch described (intellectual property); Orna Therapeutics: Consultancy, Other: Advisory board member; Chimagen: Consultancy, Other: Advisory board member; Sellas Life Sciences: Consultancy, Other: Advisory board member; Bluebird Bio: Consultancy, Other: Advisory board member; Allogene: Consultancy, Other: Advisory board member, Research Funding; Athenex: Consultancy, Other: Advisory board member; Janssen: Consultancy, Other: Advisory board member; Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; Morphosys: Consultancy, Other: Advisory board member; Kite, A Gilead Company: Consultancy, Other: Advisory Board Member, Research Funding; Fosun Kite: Consultancy, Other: Advisory board member; Carsgen: Consultancy; Longbow Immunotherapy: Current holder of stock options in a privately-held company; Precision Biosciences: Research Funding; Merck: Consultancy, Other: Advisory Board Member; Bristol Myers Squibb: Consultancy, Other: Advisory Board Member, Research Funding; Synthekine: Consultancy, Other: Advisory board member; Takeda: Consultancy, Other: Advisory board member; Adicet Bio: Consultancy, Other: Advisory board member, Research Funding; Incyte: Consultancy, Other: Advisory board member; Caribou: Consultancy, Other: Advisory board member; Sana Biotechnology: Consultancy, Other: Advisory board member, Research Funding. Vega:Allogene: Research Funding; Geron: Research Funding. Green:Sanofi: Research Funding; Abbvie: Honoraria; BMS: Consultancy; Daiichi Sankyo: Honoraria; Allogene: Research Funding; Kite/Gilead: Research Funding; Abbvie: Research Funding; KDAc Therapeutics: Current equity holder in private company. Flowers:TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Takeda: Research Funding; National Cancer Institute: Research Funding; Sanofi: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Morphosys: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding; Guardant: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; 4D: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Strati:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; ALX Oncology: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche Genentech: Consultancy; Astrazeneca Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; Hutchinson MedoPharma: Consultancy.
Lenalidomide, tafasitamab, and acalabrutinib are not indicated in 1L LBCL therapy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal